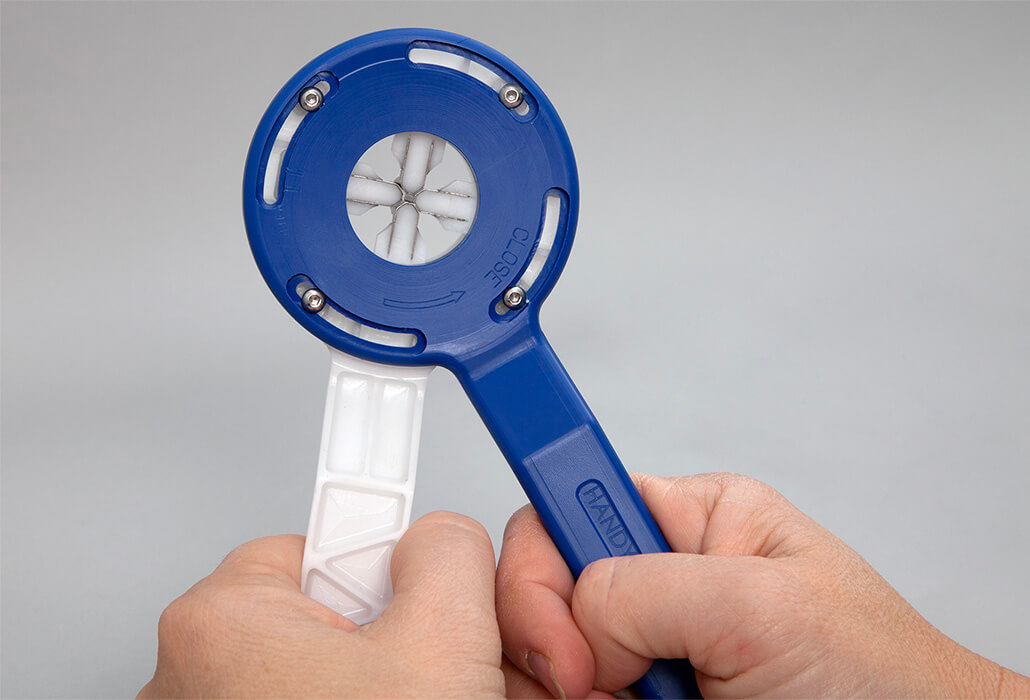
Health Enterprise East (HEE), the NHS medtech innovation consultancy, has secured an exclusive licence for the marketing of Handytrach, a pioneering device that is set to deliver safer, faster and more cost effective percutaneous dilational tracheostomies (PDTs).
All current PDT techniques pose significant risks to patients, often requiring the deployment of excessive force, which can damage the posterior wall of the trachea, as well as fracturing the tracheal rings and the surrounding tissue. In contrast, Handytrach’s novel dilation mechanism allows for a more controlled opening of the stoma thereby significantly reducing the risk of post procedural complications.
Handytrach’s innovative blade dilation mechanism evenly distributes the force to the tracheal rings and puts no additional force against the posterior wall. This also removes the need for the blind insertion of long dilators into the trachea, which has historically caused severe complications such as posterior tracheal wall perforation, anterior tracheal wall damage, and cartilage ring fracture. With some 14,000 PDTs performed annually in the UK, and a further estimated four million in the US and the EU, Handytrach’s novel mechanism has the potential to offer transformative benefits on a significant scale.
HEE supported Handytrach’s inventors, Dr. Basil Matta and Dr. Frank Rasulo at Cambridge University Hospital (CUH) NHS Foundation Trust Addenbrooke’s Hospital, throughout the early-stage development phases, including conducting a full market assessment for the device. These studies allowed the clinical team to clearly identify the market opportunity and confirmed Handytrach’s advantages over current standard practice. In addition, bench testing showed that Handytrach’s ease of use and simplified procedure could free up time for clinicians as well as reduce hospital overheads.
HEE has also helped identify JEB Technologies as the development and commercial partner for Handytrach. Based in Mildenhall, Suffolk, JEB Technologies are a well-established manufacturing company with a long history of bringing new products to market. As well as being vastly experienced in manufacturing, JEB Technologies also specialise in the design and development of novel and disruptive medical devices. With the commercial licence now in place, the JEB Technologies team are also working closely with all stake holders to further develop the technology, to take the device through clinical investigation and prepare the technical documentation for conformity assessment. Alongside this, the JEB sales and marketing team will be making product marketing preparations with a view to launching Handytrach in the UK.
Dr. Anne Blackwood, Chief Executive of HEE, said:
“Handytrach truly demonstrates the strength of innovation within the NHS right now. The team understood the clinical need to enhance PDT procedures and by accessing the right clinical and technical support, have been able to find a solution through innovation. It has been incredibly exciting to work in such close collaboration with the Handytrach team and we are delighted to reach this milestone in the device’s development journey. We look forward to supporting Handytrach through the next development phase.”
Dr. Basil Matta commented:
“PDTs are relatively commonplace procedures that have historically come with a relatively high risk of complications. What began as the germ of an idea for safer and more effective delivery of PDTs is now a reality thanks to the support from HEE . It is immensely rewarding to have developed an innovation that will soon hopefully make a profound difference to thousands of lives.”
Prof. Frank Rasulo (inventor) commented:
“Handytrach has been developed bearing in mind some of the most important and fundamental aspects related to invasive manoeuvres performed at bedside on critically ill patients within the Intensive Care Unit, rapidity and reduced invasiveness, while maintaining efficiency and safety. We’re sure that this device represents a further evolution of PDTs and will contribute to an improvement in patient care.”
Sean Licence, Business Development at JEB Technologies:
“Handytrach is a wonderful example of how a well thought out concept has the potential to significantly reduce patient complications, as well as simplify the procedure for the users. We are so happy as a company to have signed the commercial rights for this device, and we look forward to working closely with all other stake holders in bringing this technology through the design and development process, and in to the market.”
News & Analysis