Pfizer Ltd has announced that the UK Medicines and Healthcare products Regulatory Agency (MHRA) has issued an Early Access to Medicines Scheme (EAMS) positive scientific opinion for abrocitinib, an investigational treatment for people with severe atopic dermatitis requiring treatment with systemic therapy and have had inadequate response or have lost response to approved systemic therapies, or those who are ineligible or intolerant of approved systemic therapies.
The aim of the EAMS is to provide earlier availability of promising new unlicensed medicines and medicines used outside their licence, to UK patients that have a high unmet clinical need.
The positive scientific opinion for abrocitinib is based on information and clinical data relating to the benefits and risks of the medicine. It informs clinicians for their own decision making to prescribe the unlicensed treatment under their own responsibility.
Monica Nijher, Inflammation and Immunology Medical Lead, Pfizer UK commented:
“We’re delighted to announce that the MHRA has issued a positive scientific opinion for abrocitinib. The UK EAMS is a great example of how, by working together, we can provide clinicians and patients with access to innovative medicines faster in areas of high unmet need. We hope that, under the EAMS for abroctinib, those patients with the highest need – those who have a severe disease and who have run out of treatment options – can have renewed hope.”
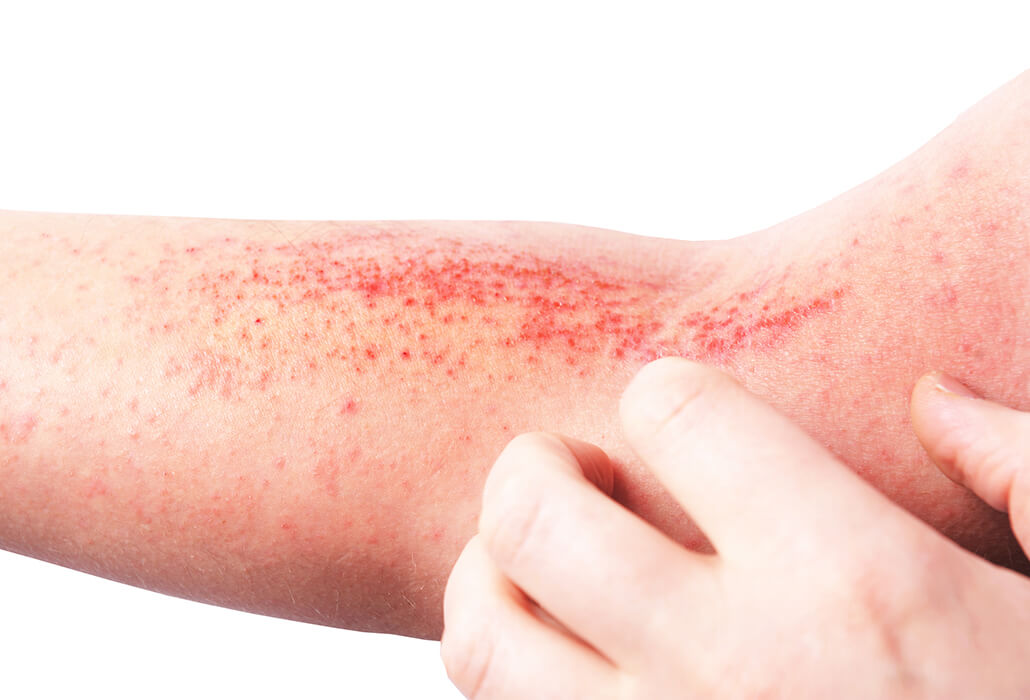
Atopic dermatitis is the most common chronic inflammatory skin disease, affecting 1 in 5 children and 1 in 12 adults in the UK. Severe atopic dermatitis is a seriously debilitating disease that has a major impact on quality of life.
Abrocitinib acts by reducing the activity of the immune system, through blocking the action of an enzyme known as Janus kinase 1.
Last year, abrocitinib received a Promising Innovative Medicine (PIM) designation from the MHRA.
Abrocitinib is not licensed in the UK to treat patients with severe atopic dermatitis and it is currently under review by regulators.
News & Analysis




