Lightpoint Medical, a medical device company developing miniaturised cancer detection tools for robot-assisted surgery, has completed an $8 million financing round. The funding includes follow-on investment from private investors, Oxford Technology, Venture Founders, Coutts Bank, and new funding from the British Business Bank Future Fund, bringing the total raised by the company to $18 million.
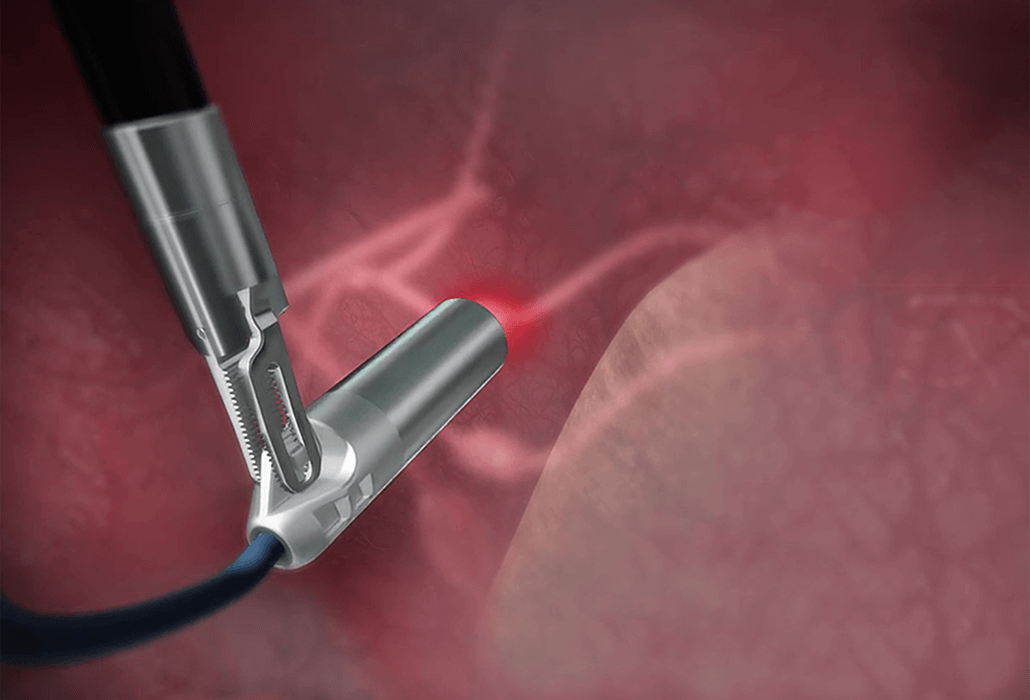
The new capital will be used to finance expanded clinical trials and early commercialisation of Lightpoint’s robotic probe, SENSEI®, which has the potential to assist in over 2 million applicable surgical procedures every year in the EU, US, China and Japan.
SENSEI received CE Mark approval at the beginning of the year, has been registered successfully with the FDA, and has received approval for sale in Australia.
Lightpoint has secured commercial distribution deals for SENSEI in Spain, Portugal, and Australia. With more contracts to be announced in the forthcoming months, Lightpoint intends to establish a global distribution network by the end of 2021.
Clinical trials of SENSEI in robotic surgery are ongoing in prostate and cervical cancer with new studies in lung cancer planned for the second half of the year.
Dr. David Tuch, CEO of Lightpoint Medical, commented:
“We’re grateful to the British Business Bank and many of our previous shareholders for investing in the round and reaffirming their commitment to Lightpoint. This fundraising follows the US and European market clearance for our SENSEI robotic probe and will enable us to further expand our clinical program and prepare for early commercial development of the technology. Our growing network of commercial distributors will help Lightpoint reach surgeons and nuclear physicians worldwide seeking a better way to perform targeted robotic cancer surgery.”
The company has also recently appointed Graeme Smith to the position of CEO to focus on the commercialisation of SENSEI. Graeme is a seasoned medtech industry executive with nearly three decades of experience successfully driving profitable business growth at global medical device companies.
Most recently, Graeme successfully led OstomyCure, an innovative medical device company developing technology to treat inflammatory bowel diseases. He will continue to serve OstomyCure as non-executive Chair.
Lightpoint’s executive leadership team includes Claire Woodthorpe, who remains as Chief Operations Officer, and new hires including Heads of Commercial Operations, Clinical, and Manufacturing.
Dr. David Tuch commented:
“The expansion of the executive leadership team at Lightpoint is central to our growth plans. As CEO, Graeme brings the ideal skills and experience needed to take our company forward as we begin to commercialize our first approved surgical probe, SENSEI. Graeme’s appointment will enable me to focus on future growth opportunities for Lightpoint. The field of nuclear medicine is at an extremely exciting stage of evolution with the emergence of highly specific cancer-targeted radiopharmaceuticals. I want to ensure that Lightpoint’s technologies are at the forefront of this revolution. I am extremely excited about our potential to transform surgical outcomes for patients across a range of major cancer types.”
Graeme Smith said:
“I am delighted join Lightpoint as CEO and look forward to working closely with David and the entire team. The company’s new miniaturized probe, SENSEI, is an incredibly exciting technology, promising accurate real-time cancer detection for minimally invasive and robot-assisted cancer surgery. I will focus initially on early commercialization of SENSEI in the fields of prostate and gynaecological cancers with an outlook to expand across a range of major cancer indications. We are aiming through our clinical program to demonstrate a significant increase in the efficacy of cancer surgery using SENSEI in combination with truly ground-breaking new cancer-targeted radiopharmaceuticals.”
News & Analysis