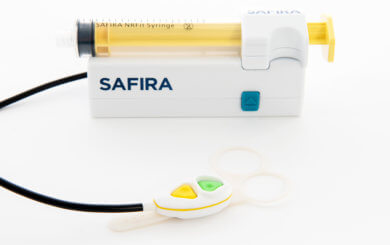
Medovate and its Brisbane-based distributor LTR Medical have secured both TGA (Therapeutic Goods Administration) Approval in Australia and inclusion on the WAND Database in New Zealand for their game-changing SAFIRA™ (SAFer Injection for Regional Anaesthesia) technology.
The regulatory approval means the UK based company – dedicated to the development and commercialisation of innovative medical technologies from within the NHS – can now start selling the pioneering device in both countries.
SAFIRA™ has been added to the TGA register for the performance of regional anaesthesia procedures. SAFIRA™ is being distributed by LTR Medical and promises a revolutionary new technology that can transform regional anaesthesia into a one-person procedure, while enhancing patient safety.
This represents the third successful regulatory approval for SAFIRA™ in 2020. The device also has FDA clearance and earlier this year received European CE Mark certification. With the use of regional anaesthesia continuing to grow significantly in Australia and New Zealand, SAFIRA™ now has the potential to make a significant impact in supporting improvements in the delivery of regional anaesthesia.
Chris Rogers, Sales and Marketing Director, Medovate, commented:
“Medovate was established three years ago with a mission to bring to life innovations with the potential to create real value by addressing unmet clinical needs. Following our partnership with LTR Medical, we are delighted to announce the successful TGA registration of SAFIRA™ and its inclusion on the WAND database. SAFIRA™ is designed to provide clinicians with an innovative solution to carry out regional anaesthesia procedures effectively at safer pressures, and we are proud to now be able to bring it to the Australian and New Zealand markets. We believe SAFIRA™ has the potential to transform the regional anaesthesia landscape and are committed to ensuring its global commercial availability.”
Danny Zanardo, VP Commercial at LTR Medical, commented:
“We are thrilled to partner with Medovate to extend the benefits of the pioneering SAFIRA™ technology to patients and anaesthetists across Australia and New Zealand. This marks another important step forward for the game-changing device, which we believe offers a powerful safety solution with potential to deliver significant value and cost savings in these regions. We look forward to a long-lasting relationship with Medovate.”
SAFIRA™ has been developed in collaboration with anaesthetists working in the UK National Health Service (NHS). The device was successfully launched in the US earlier this year, bringing to market Medovate’s first medtech innovation.
The technology incorporates a unique safety feature that helps reduce the risk of nerve damage as anaesthetic is prevented from being injected at pressures above 20psi. Furthermore, economic modelling has shown SAFIRA™ to have the potential to help deliver significant time and cost savings.
The latest certification is essential as Medovate continues to expand in territories across the US, Europe, and beyond. It marks a significant further step in making their revolutionary new medical technology available to patients and clinicians across Australia and New Zealand to help deliver improved patient care and cost saving benefits.